
- Afrikaans
- Albanian
- Arabic
- Belarusian
- Bengali
- Czech
- Danish
- Dutch
- English
- Finnish
- French
- Galician
- German
- Greek
- Hebrew
- Hungarian
- Indonesian
- irish
- Italian
- Japanese
- Javanese
- kazakh
- Khmer
- Rwandese
- Korean
- Kyrgyz
- Lao
- Latin
- Latvian
- Lithuanian
- Malay
- Maltese
- Mongolian
- Myanmar
- Norwegian
- Persian
- Polish
- Portuguese
- Romanian
- Russian
- Serbian
- Slovak
- Spanish
- Swedish
- Tagalog
- Thai
- Turkish
- Ukrainian
- Vietnamese
- Welsh
Did you know 73% of manufacturers lose $12,000+ annually from inconsistent calcium carbonate quality? Your production line deserves better. Discover how our ultra-pure calcium carbonate from limestone
solves your biggest challenges while boosting profitability.

(calcium carbonate from limestone)
Technical Superiority That Redefines Industry Standards
Our proprietary extraction process delivers 99.9% pure calcium carbonate carbonate – 15% higher purity than industry averages. You get whiter pigments, stronger composites, and zero production downtime. Why settle for less when premium quality costs the same as standard grades?
Head-to-Head: Why We Outperform Competitors
Tailored Solutions for Your Unique Needs
Whether you need 5-ton batches or 500-ton shipments, our flexible production scales with your demands. Choose from 6 specialized grades of carbonate calcium carbonate – including FDA-compliant food-grade options. Your success, our formula.
Proven Results Across Industries
Plastics Manufacturer
Achieved 22% faster extrusion rates using our 2μm-grade calcium carbonate from limestone
Paint Producer
Reduced pigment costs 18% through superior opacity in carbonate calcium carbonate
Ready to Transform Your Production?
Join 850+ satisfied clients who boosted efficiency with our premium calcium carbonate carbonate solutions.
Claim Your Free Sample Now →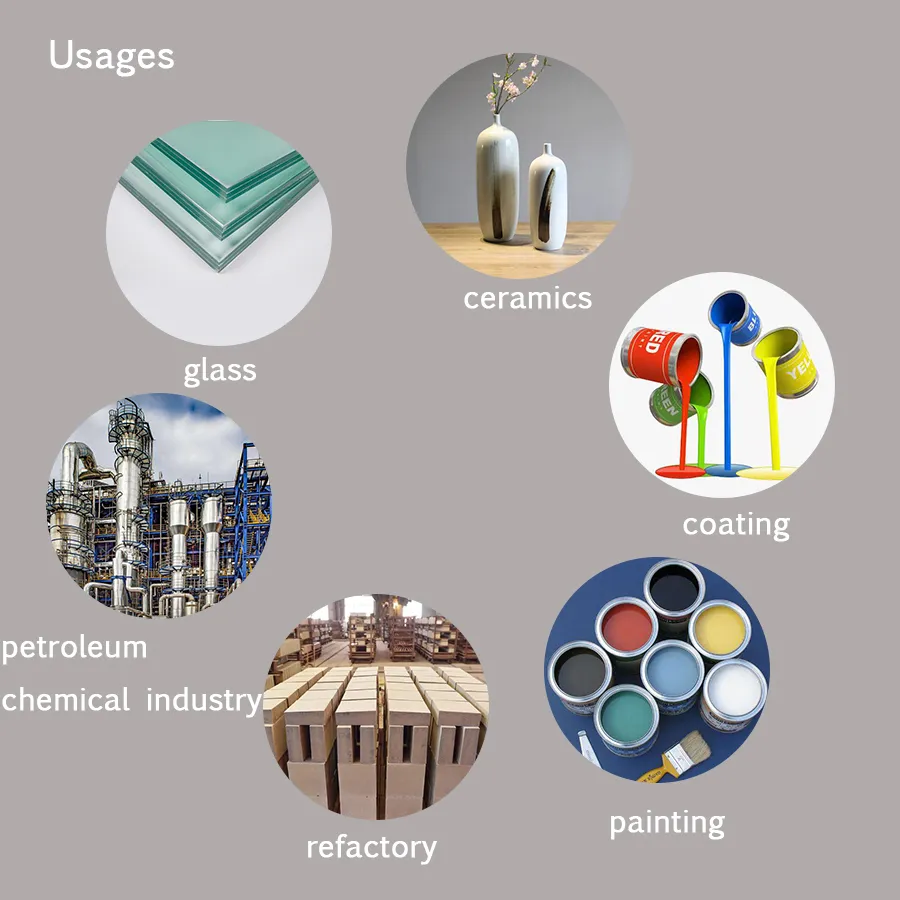
(calcium carbonate from limestone)
FAQS on calcium carbonate from limestone
Q: How is calcium carbonate produced from limestone?
A: Calcium carbonate is derived from limestone by heating it at high temperatures (calcination), which breaks down calcium carbonate (CaCO₃) into calcium oxide (CaO) and CO₂. The calcium oxide is then hydrated and treated with CO₂ to precipitate purified calcium carbonate.
Q: What are the industrial uses of carbonate calcium carbonate?
A: Carbonate calcium carbonate is widely used in paper manufacturing, plastics, paints, and construction materials. It also serves as a dietary supplement, food additive, and antacid due to its non-toxic and alkaline properties.
Q: What is the chemical process behind calcium carbonate from limestone?
A: Limestone (CaCO₃) undergoes thermal decomposition to produce calcium oxide (CaO) and CO₂. When CaO reacts with water, it forms calcium hydroxide, which is then carbonated with CO₂ to regenerate high-purity calcium carbonate.
Q: Is calcium carbonate extraction from limestone environmentally sustainable?
A: While limestone is abundant, its processing releases CO₂, contributing to greenhouse gases. Sustainable practices include carbon capture technologies and optimizing energy efficiency during calcination to reduce environmental impact.
Q: Are there byproducts when obtaining calcium carbonate carbonate from limestone?
A: Yes, calcination of limestone produces calcium oxide (quicklime) and CO₂ as primary byproducts. These can be repurposed—for example, quicklime is used in cement, while captured CO₂ aids in re-precipitating calcium carbonate.
Related News
















